Modulating the growth of engineered tissues
- Cell differentiation is critical in determining the structure of in vitro cultivated tissues .
- Boundary and initial conditions also play important roles in determining their growth rates and final structure.
Tissue growth in biomimetic scaffolds is strongly influenced by the dynamics and heterogeneity of cell populations. A significant source of heterogeneity is the depletion of nutrients and growth factors due to transport limitations. Cells slow down, stop dividing or even die when the concentrations of key nutrients and growth factors drop below certain levels in the scaffold interior. As a result, tissue engineers have not yet been able to grow in vitro tissue samples thicker than a few millimeters for metabolically active cells.
We have developed a multi-scale, hybrid framework that integrates biology with mathematical, computational, and experimental tools to study heterogeneous cell populations growing in three-dimensional scaffolds. We use a discrete, stochastic model to describe the population dynamics of migrating, interacting, and proliferating cells. The diffusion and consumption of nutrients and growth factors are modeled by partial differential equations that are subject to boundary conditions appropriate for the bioreactors used in each case. These PDEs are solved numerically and the computed concentration profiles are fed to receptor-mediated binding/trafficking models or simplified kinetic expressions (i.e. Monod kinetics) to modulate cell proliferation rates and migration speeds. To meet the significant computational requirements of this model, parallel implementations of the hybrid algorithms have been developed for computer clusters.
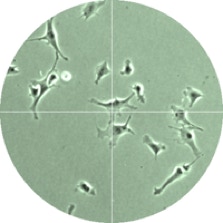
Finally, video microscopy and digital image analysis are used to experimentally observe the dynamic behavior of cell populations and find how cell migration and proliferation are influenced by the concentrations of nutrients and growth factors in the culture media, as well as by cell-substrate interactions.
Read more…
Computational Modeling for in vitro Tissue Cultivation